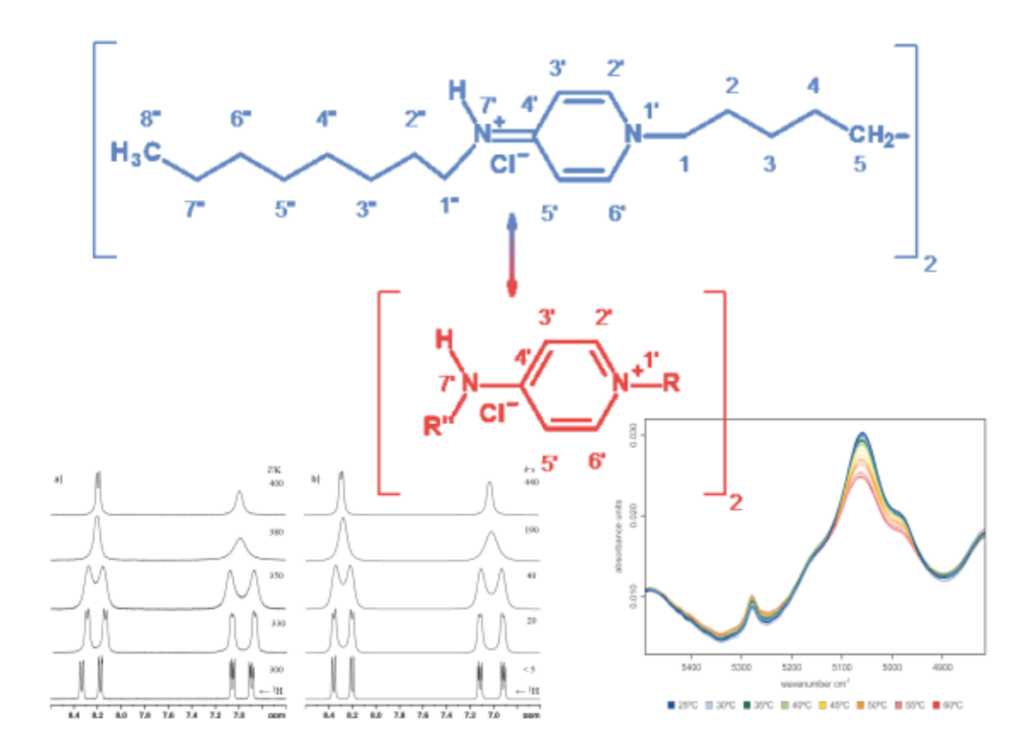
Temperature dependent analysis of Octenidine (N,N´-(decane-1,10-diyldipyridin1-yl-4-ylidene)dioctan-1-amine) dihydrochloride by NMR and NIR spectroscopy
Abstract
Octenidine dihydrochloride is one of the most common active pharmaceutical incredients with a disinfectant effect on superficial wounds. In this paper we describe the mesomeric behaviour of Octenidine dihydrochloride by comparison with 1H, 13C and 15N NMR shift data. With various NMR experiments, the possible mesomeric structures are studied and it is shown that an E/Z-isomerization plays a role, corresponding to a hindered rotation of the heteroaromatic pyridine ring. In this context, NIR spectroscopy and principal component analysis emphasize the occurrence of a modified temperature-dependent spectral behaviour of Octenidine dihydrochloride. In addition, we made a detailed study of the NMR-characteristics of Octenidine dihydrochloride using 1D- and 2D-experiments.